Research Interests
 Multigene families of Ig domain-containing innate immune receptors
Multigene families of Ig domain-containing innate immune receptors
The genomes of teleost fish, including zebrafish, possess many multigene families that encode cell surface receptors involved in innate immunity. The majority of these receptors transduce either inhibitory or activating signals across the cell membrane and utilize different types of extracellular Ig domains for ligand recognition that, unlike Ig domains involved in adaptive immunity, do not undergo somatic recombination. Several of these innate immune receptors appear restricted to the Osteichthyes class of fish. The immunological needs of these fish species, which are egg-laying with ex-utero embryonic development, may be related with the emergence and expansion of these gene families. These multigene families include novel immune-type receptors (NITRs), which have been proposed as NK receptors, diverse immunoglobulin domain containing proteins (DICPs) and polymeric immunoglobulin receptor (pIgR) like (PIGRL) proteins. Select DICP and PIGRL Ig domains proteins bind different phospholipids, an ability shared with the mammalian CD300 and TREM family of receptors. In addition, some of these gene families are located in areas of the zebrafish genome that exhibit copy number variation (CNVs). The presence of CNVs in immunological genes has been related with either resistance or susceptibility to different infections and autoimmune diseases.
It is evident that many more multigene receptor families remain to be discovered and much work is left to fully understand these families. Research into these gene families will provide a better understanding on alternative pathways of innate immunity and cross species analyses.
Immunotoxicology
Conserved innate immune response genes
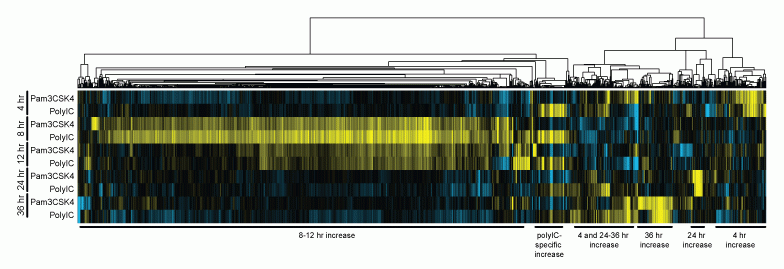
Although humans and zebrafish have several hundred million years of evolutionary divergence between them, they still share many conserved, homologous genes. The immense evolutionary pressure to maintain the homology among these conserved genes suggests that they carry vital functions in the vertebrate system. Deciphering these functions in the zebrafish model carries the potential of translational applications including identifying new therapeutic targets in human and animal disease. With this in mind, one focus of the lab is to identify and understand novel immune related genes that are maintained across multiple species. Using the zebrafish model and gene expression analyses, we have defined a whole-organism innate immune response transcriptome and identified multiple genes that are transcriptionally up-regulated in zebrafish larvae after immune stimulation with bacterial and viral mimics. Ongoing investigation and characterization of these novel genes is an exciting area of interest that garners the potential of advancing our understanding of the innate immune system as well as identification of some translational targets of future pharmaceuticals.

Zebrafish MHC Class I
The major histocompatibility complex class I (MHCI) genes play a central role in vertebrate immunity. Classical MHCI proteins are expressed on nearly all cells and present peptide antigens to cytotoxic T-cells in order to initiate an immune response. In addition, the presence of MHCI molecules on the cell surface provides a protective mark of “self” to surveillant cytotoxic (natural killer) cells. While zebrafish are known to express orthologs of mammalian MHCI, the characterization of the genetic and functional diversity of zebrafish MHCI genes is incomplete. Zebrafish MHCI genes are classified into three different lineages: the “U” genes on chromosome 19, the “Z” genes on chromosomes 1 and 3, and the “L” genes on chromosomes 3, 8, and 25. The U genes possess features characteristic of classical MHCI function, the L genes exhibit features of non-classical MHCI function, and the Z genes display features of both classical and non-classical MHCI. With the availability of individual animals from diverse genetic backgrounds, both laboratory-raised and wild-caught, zebrafish are a great model for investigating the intraspecific diversity of this highly polymorphic and rapidly evolving multi-gene family. Zebrafish studies are being partnered with cell-based studies to determine the functional diversity of the different MHCI lineages in zebrafish.